DetTX: Engineering Excellence for the Defense Industrial Base
DetTX is an independent engineering and design firm with over four decades of expertise
in energetic materials for large-caliber ammunition and weapon systems. We have led the
design, construction, commissioning, and optimization of munitions manufacturing facilities
across the United States and worldwide, including 155mm artillery melt-pour plants,
pressed warhead lines, propellant manufacturing sites, and demilitarization facilities.
We provide technical leadership at every stage of the facility lifecycle, from site
planning and process design to environmental and explosive safety compliance, commissioning,
and operational optimization. Our proven track record ensures throughput, quality, and
safety from day one.
At the core of our approach is a deep commitment to mission readiness. We understand that
every project we execute directly supports the men and women who serve.
Our priorities are:
Our legacy is defined by the facilities we build and the capabilities they deliver. At DetTX, we do more than design systems, we engineer readiness.
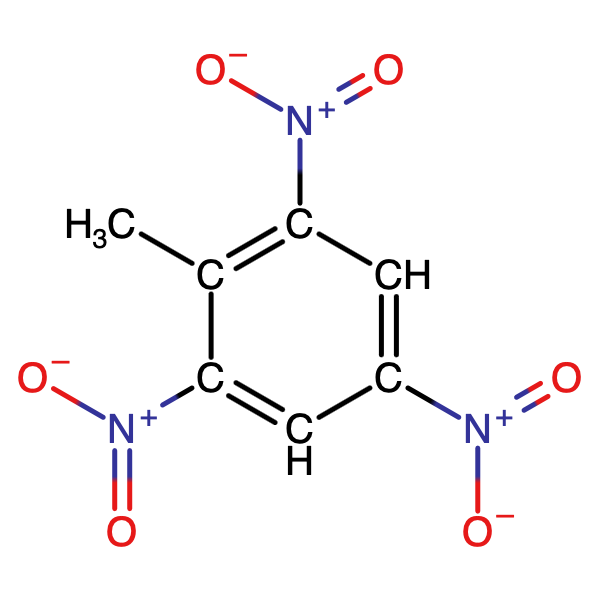
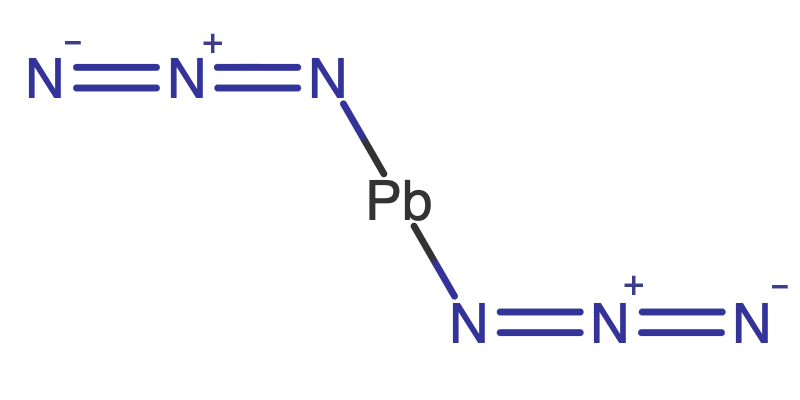
Pure explosive compounds are listed in Explosive Compounds
along with many of their important properties. TNT is one of the few energetic materials that can be used, as is, in artillery,
mortar and demolition charges2 without additional ingredients. However, most high explosives are mixtures of
explosive compounds, fuels, oxidizers, binders and other additives, these formulations are
listed in Explosive Formulations.
1 ΔHc Butter = 30.5 kJ/g compared to ΔHc TNT = 15.0 kJ/g
2 TNT melts at 80.4°C (178°F) which allows it to be melted in water jacketed vessels and then frozen into a solid for operational use.
Last update August 14th 2025 - Links to Formulations, Compounds and Glossary added
Explosive materials (explosives, explosive compositions, pyrotechnics, gun propellants , rocket propellants) are extremely dangerous and should only be prepared, handled and tested by experts who are skilled in the art. The information on this website has been collected from a large number of reliable references. However, DetTX cannot be held liable in any way for any damage to persons or property relating to the misuse of the information on this website Under federal explosives law, it is illegal to manufacture, store, distribute, receive or transport explosive materials without a federal explosives license or permit (FEL/FEP).