DetTX provides independant consultancy services to the defense industry in the field of explosive compounds, their synthesis and processing.

This page will be under development for ever
| Nominal burning rates (m/s)-1 | ||||
|---|---|---|---|---|
| Low Explosives | High Explosives | |||
| Rocket | Gun | Sound speed | Primary | Secondary |
| 0.001 | 0.05 - 0.5 | 335 | 5,000 | up to 10,000 |
Nitrocellulose is continually degrading, losing nitrogen oxides from the nitro groups. This process cannot be prevented and means that nitrocellulose based propellants have a shelf life. The nitrogen oxides that are formed by this degration process have an autocatalytic effect on the degradation reaction. Unchecked this reaction can lead to spontaneous combustion of Nitrocellulose. Stabilizers absorb the Nitrogen Oxides produced to form nitrate or nitroso groups within their structure

|
Arkadite II (Methyl Diphenyl Urea) Biuggl gjglglg lgll utdtryl gku fk fkfkj kjkjh fkjfk yfuc kjlko iu guyftr sy trdy tfvu tyj ytgh rfytfh fhf |

|
Methylnitroaniline Biuggl gjglglg lgll utdtryl gku fk fkfkj kjkjh fkjfk yfuc kjlko iu guyftr sy trdy tfvu tyj ytgh rfytfh fhf |

|
2-Nitrodiphenylamine Biuggl gjglglg lgll utdtryl gku fk fkfkj kjkjh fkjfk yfuc kjlko iu guyftr sy trdy tfvu tyj ytgh rfytfh fhf |

|
DNT (2,4-Dinitrotoluene) |

|
Diphenylamine |

|
Ethyl Centralite |

|
Methyl Centralite |
Like the Butler in Agatha Christie novels when there is an explosive accident it is almost always the Nitrocellulose that did it. The reason is that Nitrocellulose is continually degrading and releasing nitrogen oxides which autocatalyzes the degradation reaction. Stabilizers in the propellants absorb these nitrogen oxides but eventually get used up after which it is only a matter of time before the propellant catches fire and deflagrates. The early history of the development of nitrocellulose is a series of factory explosions.
A CAS Registry Number, also referred to as CASRN or CAS Number, is a unique numerical identifier assigned by the Chemical Abstracts Service (CAS) to every chemical substance described in the open scientific literature (currently including all substances described from 1957 through the present, plus some substances from the early or mid 1900s), including organic and inorganic compounds, minerals, isotopes, alloys and nonstructurable materials (UVCBs, of unknown, variable composition, or biological origin)
(Explain CAS validation)
Deflagrate combines the Latin verb flagrare, meaning "to burn," with the Latin prefix de-, meaning "down" or "away." A deflagration is a very rapid burning where heat and large volumes of gas are generated. The reaction procedes by the passage of a flamefront through the material. The burning rate is often accelerated by increasing the pressure as the combustion zone near the burning surface becomes compressed helping heat conduction to the surface but will remain below the speed of sound in the solid. Whether or not an explosion occurs as a result of a deflagration depends on confinement. This confinement may be a physical barrier or if the quantity of material is sufficient the mass of the material and the force required to accelerate it can become a virtual confinement. In many cases without confinement a deflagration will not take place and the material may just burn quickly. Materials that deflagrate are also known as Low Explosives.
Detonate from Latin detonare to thunder away, from de- + tonare to thunder. In a detonation the explosive, which has both the fuel and oxidizer together on a molecular level, is initiated by a shockwave. There is no flame front, the reaction takes place at the leading edge of the shockwave and proceeds at a speed higher than the speed of sound in the unreacted material. The shockwave disassociates the molecule into fragments that react together rapidly to create high pressure and temperature to give further impetus to the shockwave and maintain its propagation. In a detonation the reaction zone is supersonic i.e. higher than the speed of sound in that solid. Detonation velocities are several orders of magnitude greater than for a deflagration, see burning rate. Explosives that can sustain a detonation are also known as High Explosives.
An explosion may be defined as a loud noise
accompanied by the sudden going away of
things from the places where they were before.

Explosophores are functional groups in organic chemistry that give organic compounds explosive properties. Common functional groups and structure are shown below.
(Add group additive enthalpies to each group below)
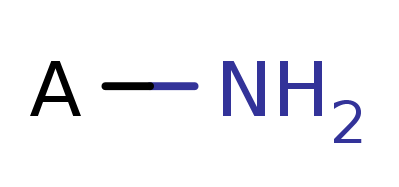
Amine
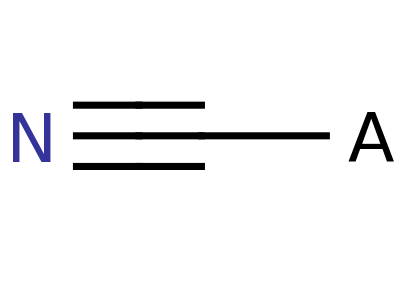
Nitrile
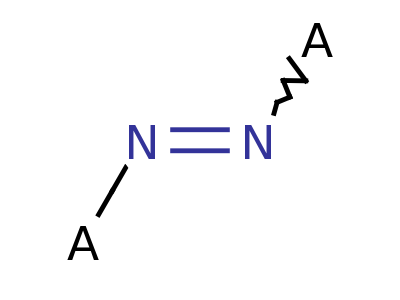
Diazo
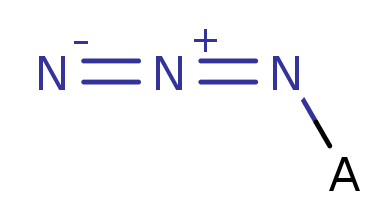
Azido
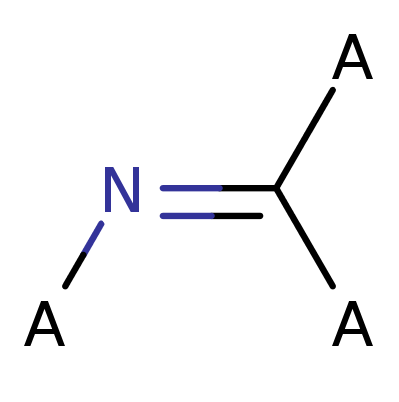
Imine
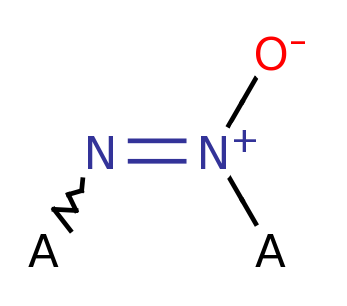
Azoxy

Ether
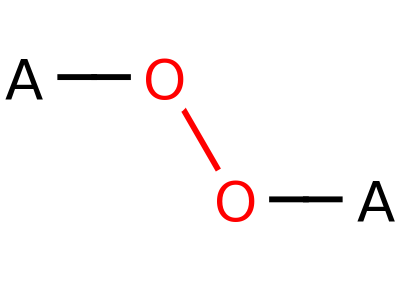
Peroxide
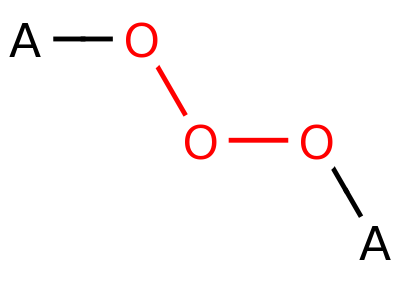
Ozonide
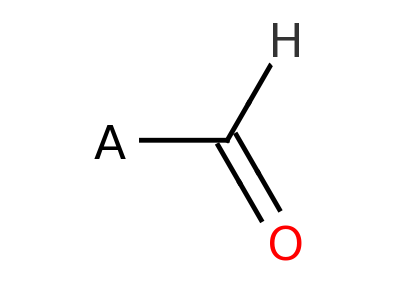
Aldehyde
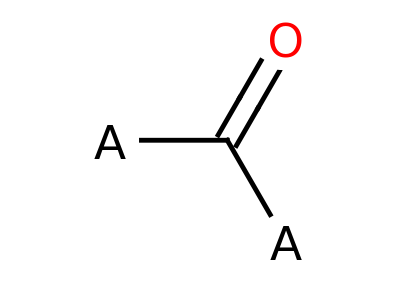
Ketone
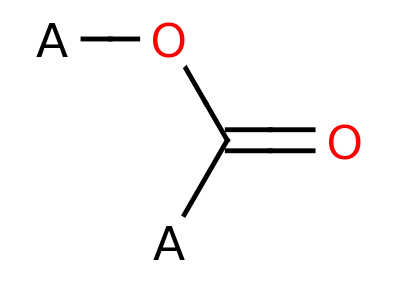
Ester
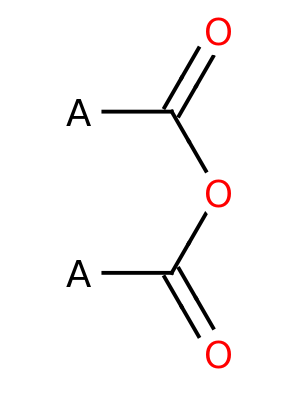
Acid anhydride
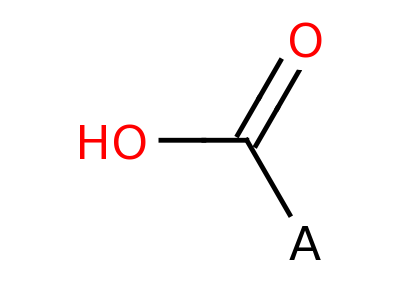
Carboxylic Acid
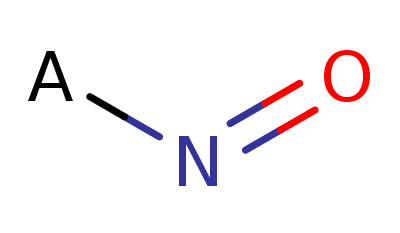
Nitroso
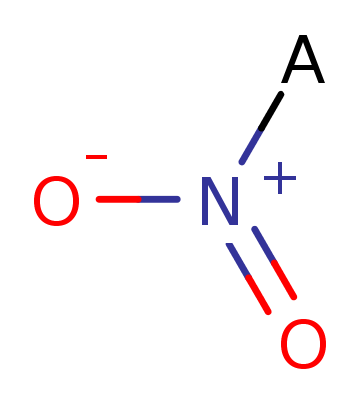
Nitro

Nitrato
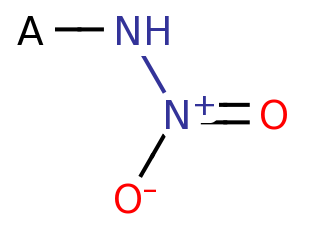
Primary Nitramine

Secondary Nitramine
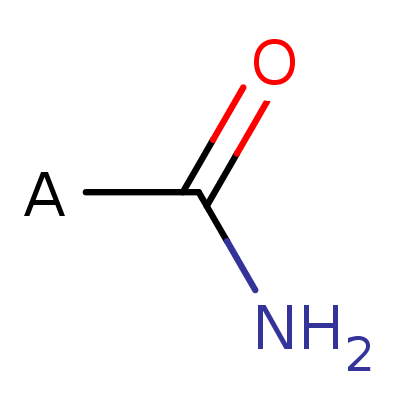
Amide
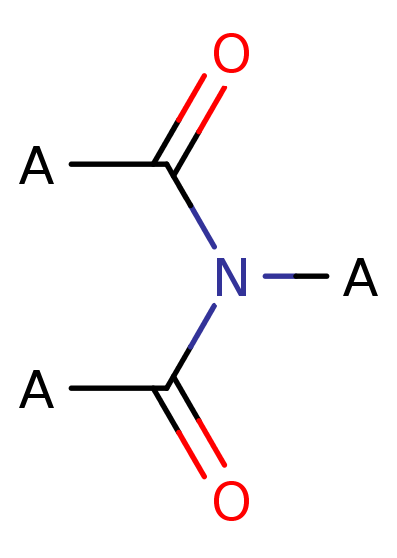
Imide
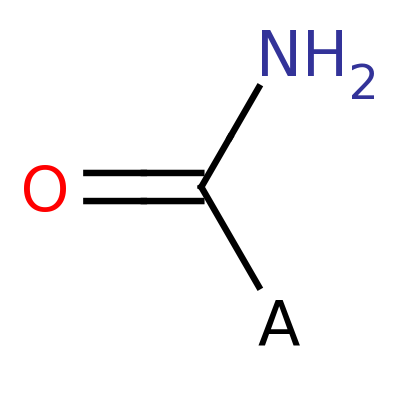
Carboxamide
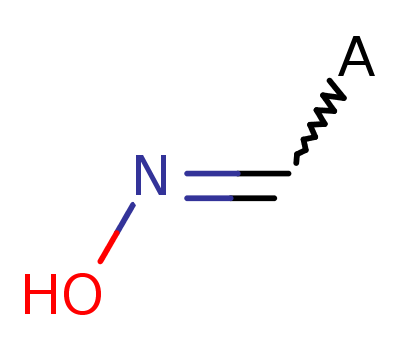
Oxime
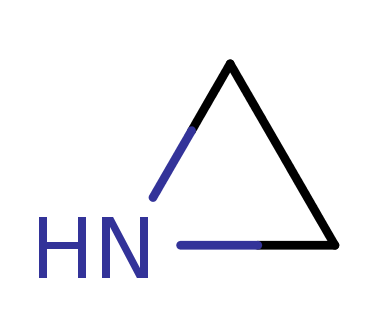
Aziridine
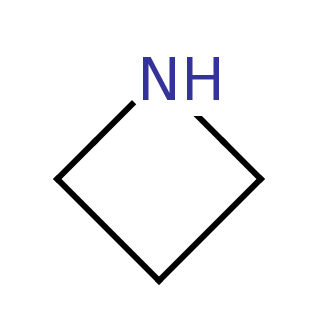
Azetidine
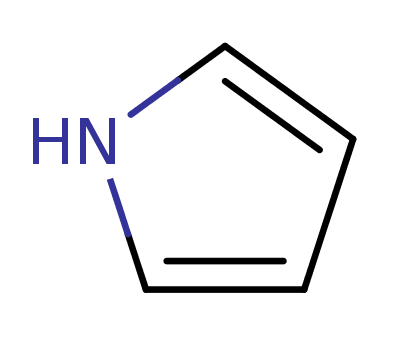
Pyrrole
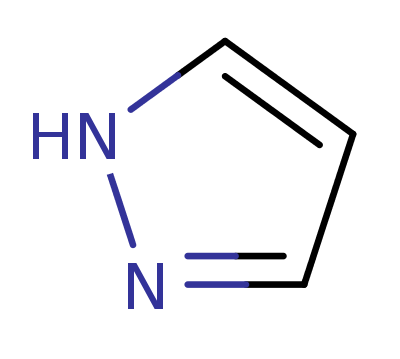
1H-Pyrazole
(1,2-Diazole)
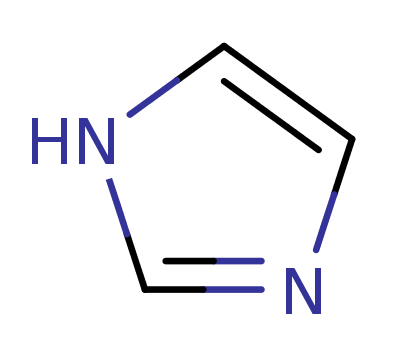
1H-Imidiazole
(1,3-Diazole)
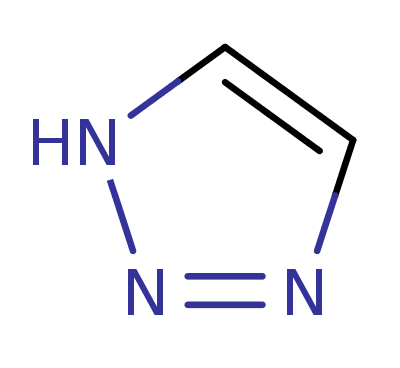
1H-1,2,3-Triazole (Triazoline)
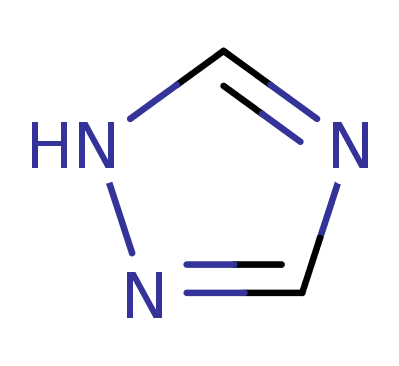
1H-1,2,4-triazole
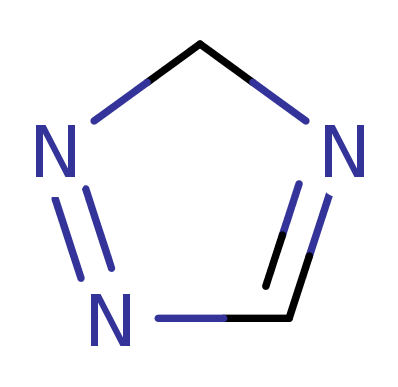
3H-1,2,4-Triazole

Tetrazole
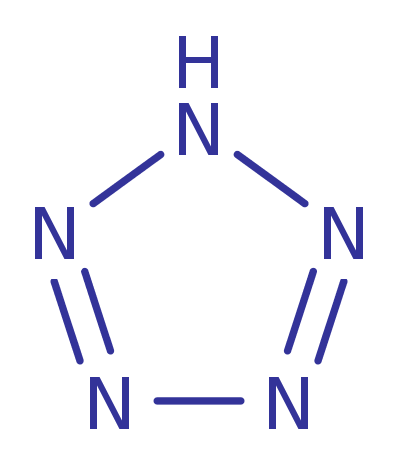
1H-Pentazole
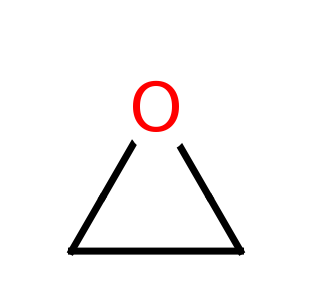
Oxirane
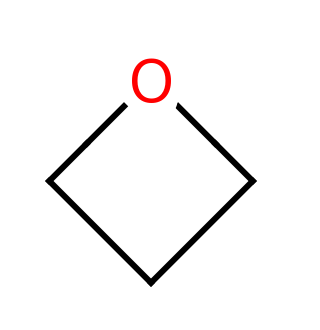
Oxetane
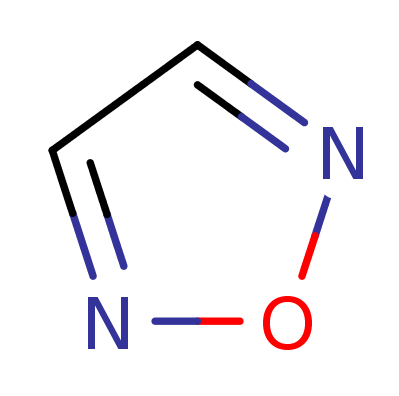
1,2,5-Oxadiazol
(Furazan)
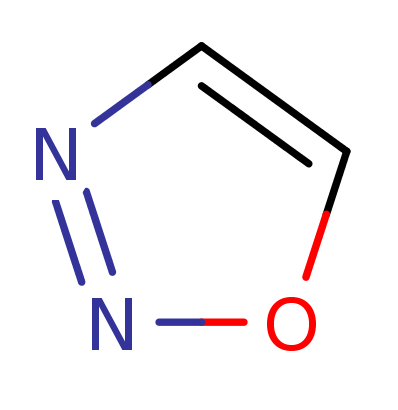
1,2,3-Oxadiazol
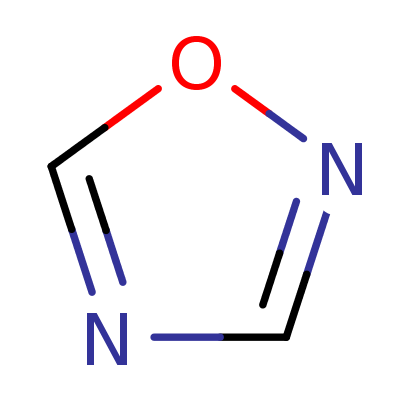
1,2,4-Oxadiazol
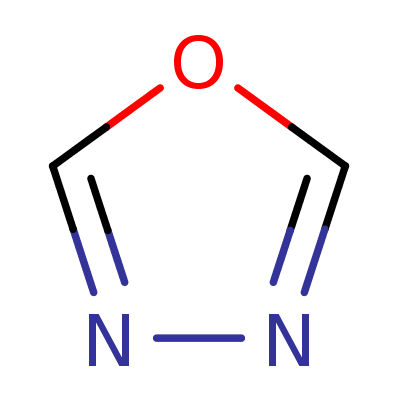
1,3,4-Oxadiazol
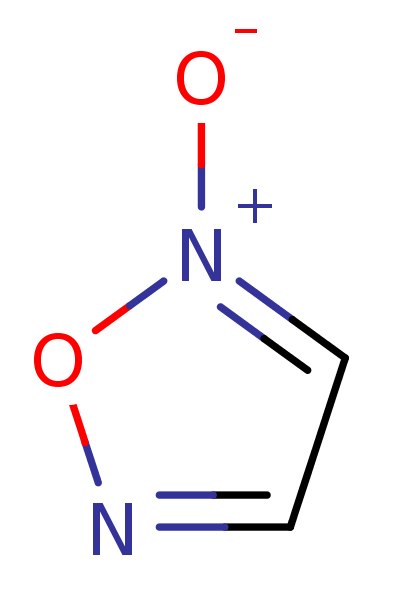
Furoxan
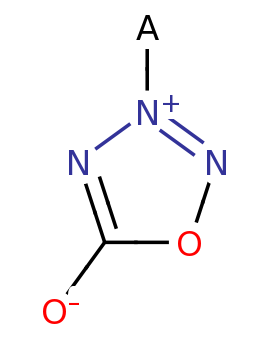
Azasydnone
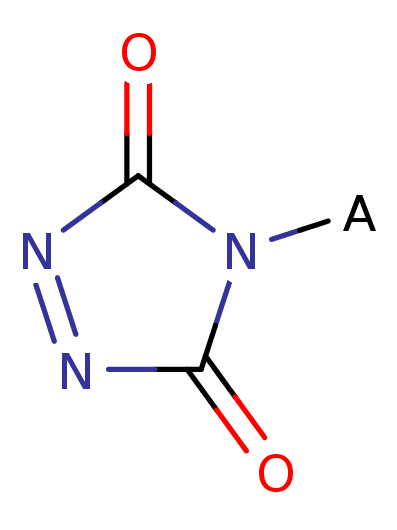
A 1,2,4-triazole-3,5-dione

Benzene
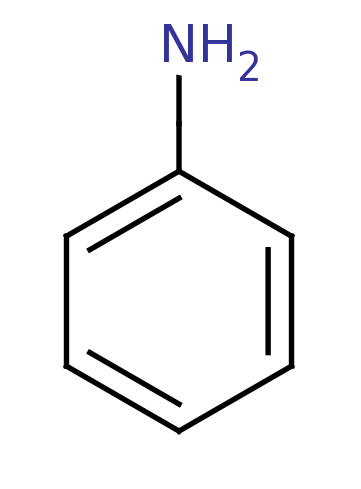
Aniline
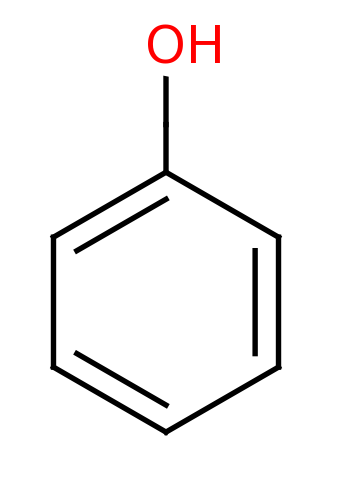
Phenol

Resorcinol

Toluene
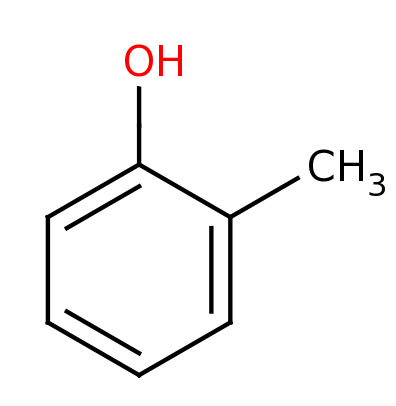
o-Cresol
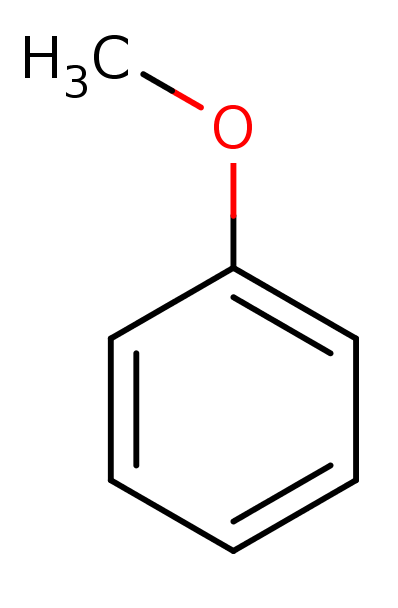
Anisole
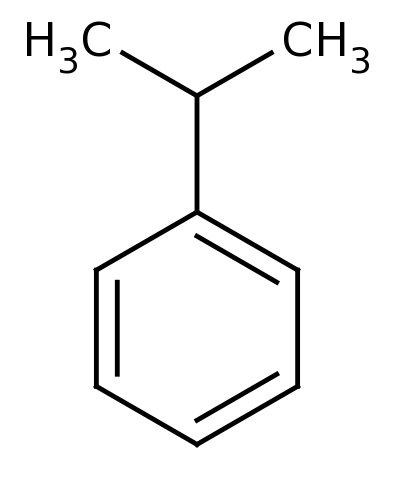
Cumene
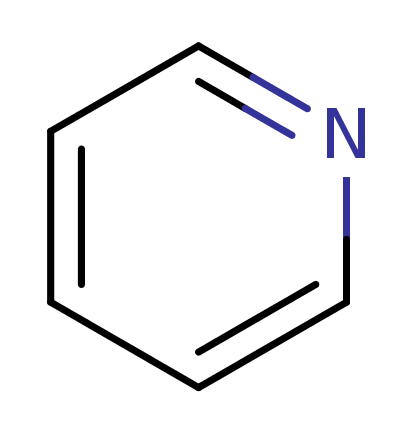
Pyradine
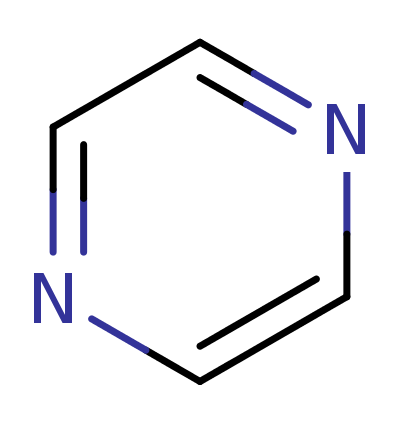
Pyrazine
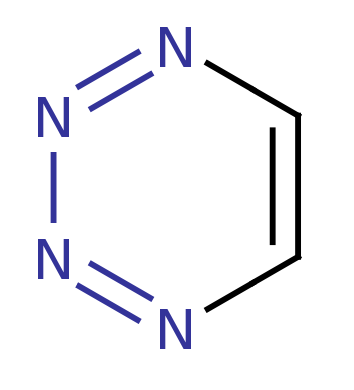
1,2,3,4-Tetrazine
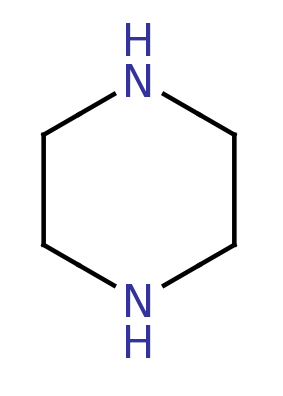
Piperazine
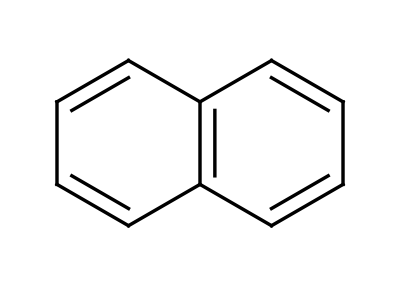
Napthalene
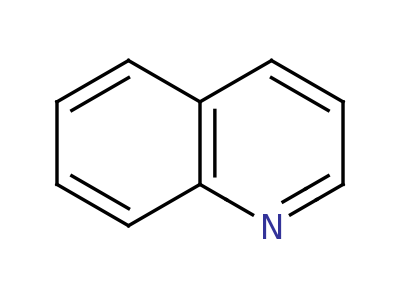
Quinolene
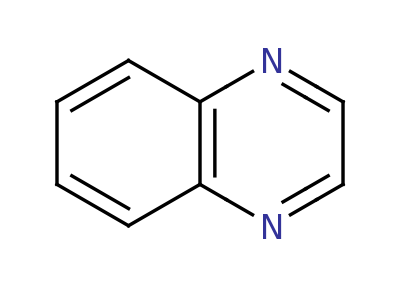
Quinoxaline
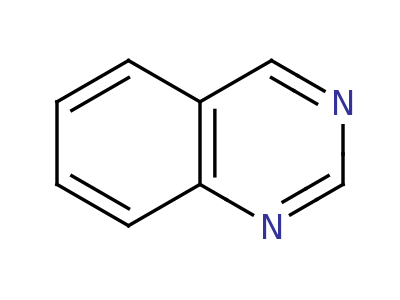
Quinazoline
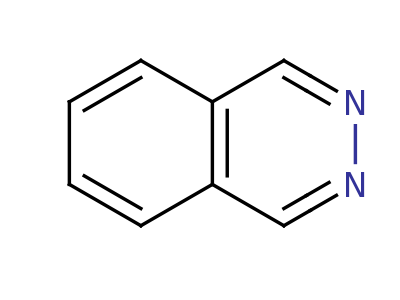
Phthalazine
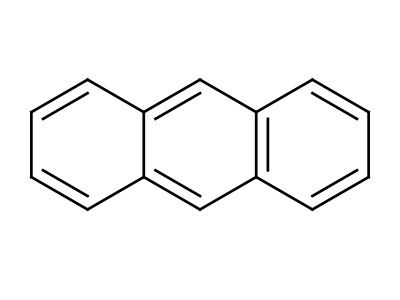
Anthracene
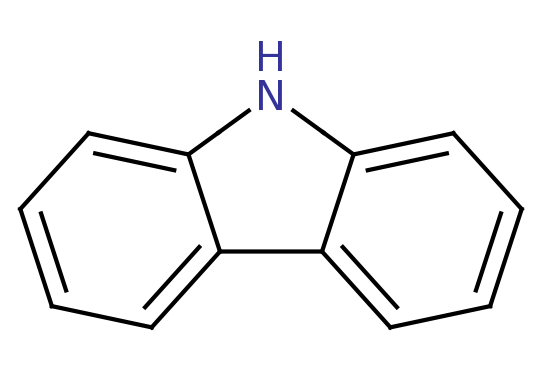
Carbazole
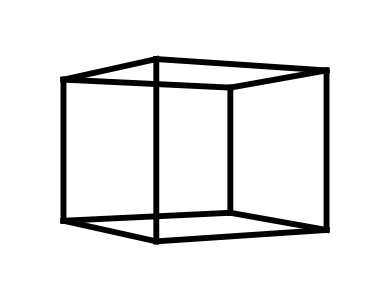
Cubane

Tri-nitro Chickenwire